Basically there are two distinctively types of unit cell in constructing the lattice: primitive and non-primitive unit cells. First, let us take a look a unit cell on the Figure 1a, which is the example of primitive unit cell. If we look closely, the total part of the lattice points (from each corners) which contributes in forming the primitive unit cell is one, as illustrated from Figure 1b.
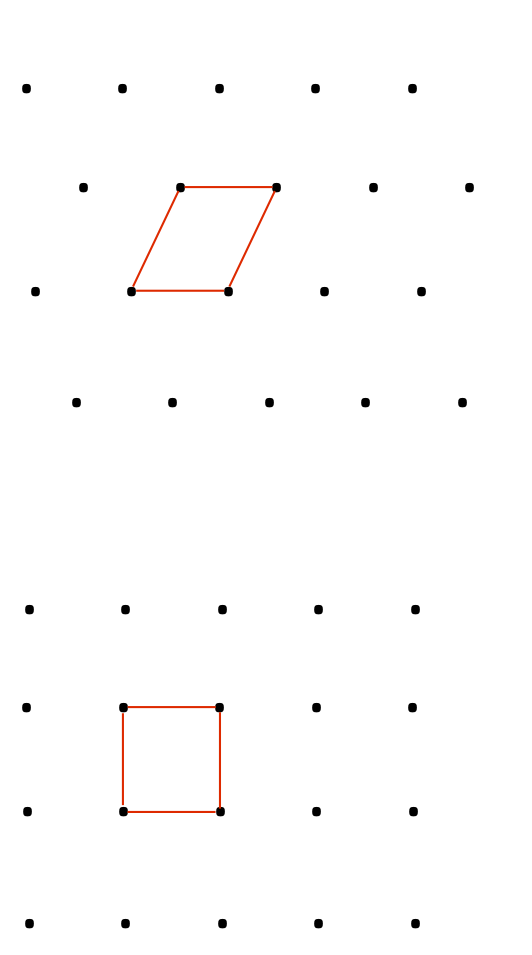
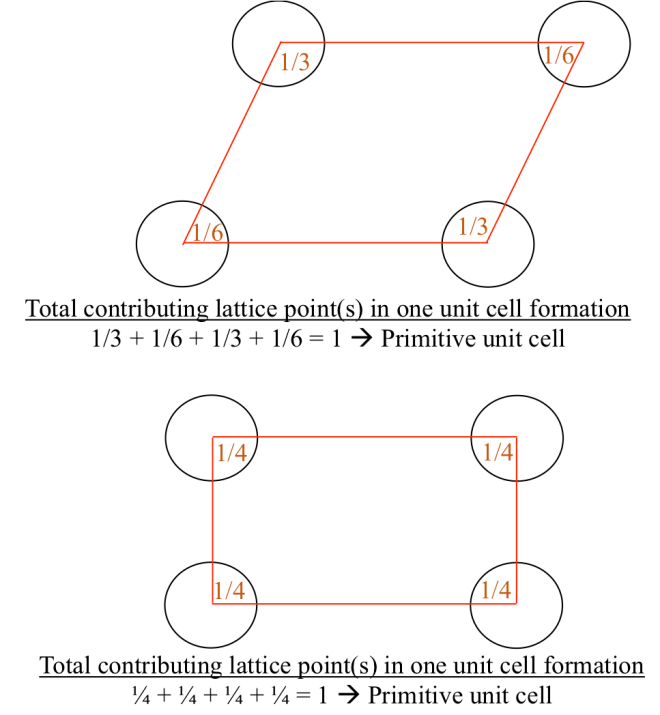
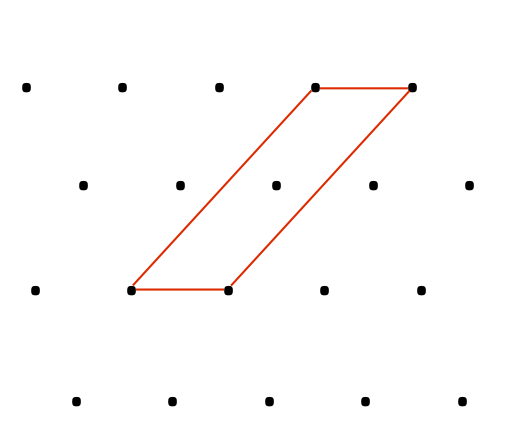
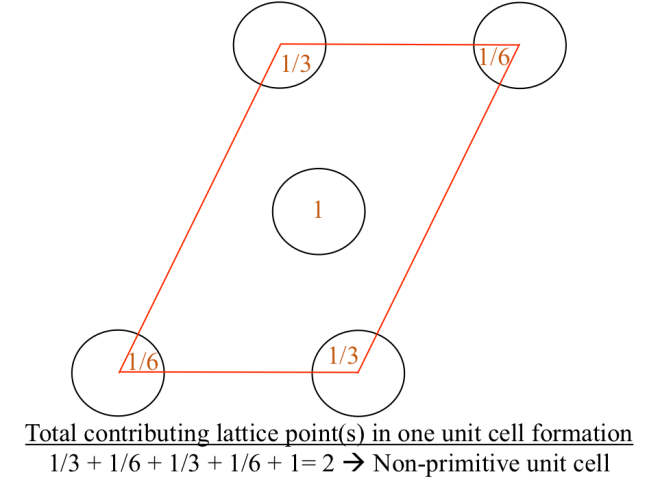
Let us now consider unit cell in Figure 2a. We notice there is another one lattice point inside the unit cell and it sums to be two lattice points (Figure 2b) in building this unit cells. Generally, for the type of unit cell which possess more than one lattice points is called non-primitive unit cell. Therefore, we can distinguish whether the unit cell is primitive or non-primitive by counting the number of lattice point contributing to one unit cell, regardless the shape of that unit cell.
Determining the lattice whether it is composed of primitive or non-primitive unit cell can be also have the same criteria as it has described earlier: The symmetry of unit cell -> having higher symmetry is more likely to be real unit cell, and The volume of unit cell -> having smaller volume is more likely to be real unit cell. To understand this, let us consider lattice point in the Figure 3a. Next, we can draw parallelograms in this lattice to form possible unit cells, shown in Figure 3b.
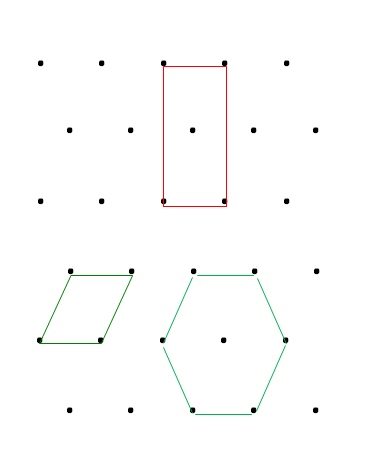
As we can see from Figure 3b, at least there are three possible parallelograms drawn on the available lattice point. The rectangular (green) is chosen as the unit cell because it has the smallest volume while at the same time possesses the most symmetry compared to other hexagonal and parallelogram (red). The symmetry in here includes rotation and reflection symmetry. This result implies that it is not always the smallest unit cell chosen as the unit cell, but it must also contain largest possible symmetry. Therefore, the unit cell composing this lattice is non-primitive.
Let’s say we have different arrangement of lattice, as it is illustrated in Figure 4a, which is more compact than lattice point in the Figure 3a. As usual, we try to find possible parallelogram for this unit cell 4b. We found out the unit cell for this type lattice arrangement is hexagonal unit cell (red), for it is having 6-fold symmetry rotation and 6 symmetry reflection. The rectangular one does not longer fulfill the criteria as unit cell, not as capable as the hexagonal one. Since this unit cell has two lattice points in it, we can say that hexagonal unit cell in this lattice arrangement is non-primitive.


Now I think we are done with 2D and have enough understanding to move further to 3D lattice with its 3D unit cell.
Further reading: http://www.doitpoms.ac.uk/tlplib/crystallography3/unit_cell.php